 by Autumn Ryan, Founder and CEO, Aseptic Health
by Autumn Ryan, Founder and CEO, Aseptic Health
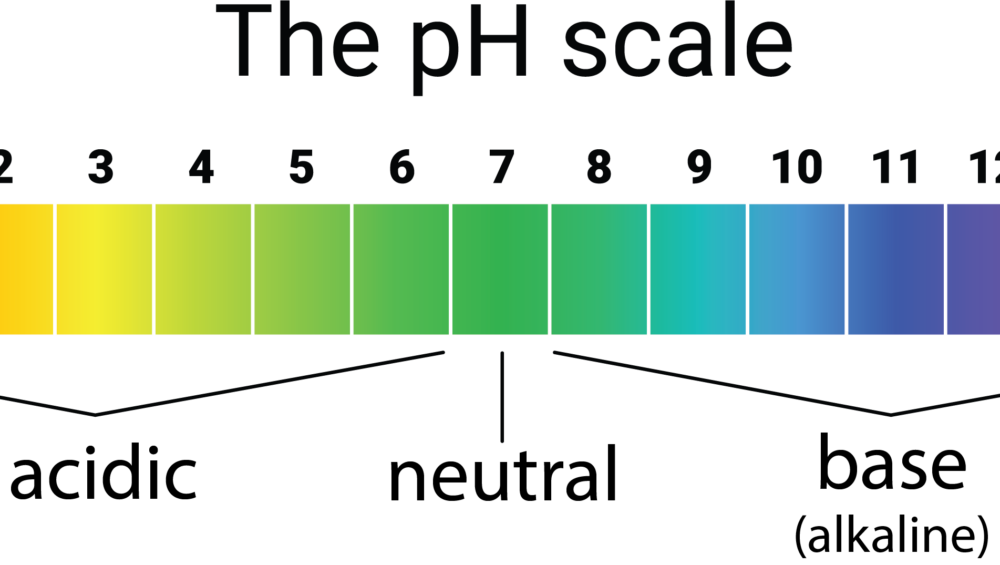
What does it mean to have a neutral pH? Neutral solutions have a pH of 7.0. Water is an excellent example of a neutral solution. Add a solution to water and the pH (or potential of hydrogen) changes. Your lemonade becomes acidic. Your bicarbonate of soda becomes alkaline or basic.
The pH Scale
The pH scale goes from 1 to 14 with 7 being neutral. Anything below 7 is considered to be acidic. Anything above 7 is considered to be basic. Because it is a logarithmic scale, each number is 10 times more powerful or less powerful than the next number. A pH of 8 is 10 times more basic than water and a pH of 9 is 100 times more basic and so on.
Extreme pH on either end can cause severe harm. Alkali burns to the eye are worse than acidic burns because substances that cause alkali burns contain lye, lime or ammonia. Think liquid drain cleaner. But you can also get severe burns from acidic chemicals. Vinegar, which is a weak acid, wouldn’t do much harm, but car battery acid, which is a strong acid, will.
Buffer Solutions
Buffer solutions are often used in the cleaning industry to manage the pH of water to resist change upon adding the acidic or basic ingredients necessary for adequate cleaning.
Why Know The pH Of Cleaning Solutions?
The reason it is important to know the pH level of the cleaner you’re using is that the pH can have an effect on the material you’re cleaning. Strong acidic or strong basic cleaners can cause fabric dye to run or its sheen to be affected. Cleaners that work effectively that are closer to the neutral scale are considered safer to use with all materials. [For those in the soft surface cleaning industry, Cleanfax magazine published an excellent article in April, 2020 on The Role of pH in Cleaning.
For household cleaning, remember basic solutions are used for cutting through dirt, grease, proteins, oils and other organic items. Acids are typically used for removing calcium, rust and other minerals. But there are some very effective cleaners that come closer to neutral. Dish soaps, for example, are usually in the pH range of 7 to 8.
Let’s take a look at some other common household cleaners and their pH levels.
Chlorine Bleach, pH 11 to 13
Ammonia, pH 11 to 12
Borax, pH 10
Baking Soda, pH 8 to 9
Vinegar, pH 3
Because chlorine bleach and ammonia are highly basic, we know that they are corrosive and should be used in a well-ventilated room. Also bleach and ammonia are not safe to be mixed with other ingredients.
Borax is often used as a laundry booster to break down oils and organic dirt. At a 10 on the pH scale it is still corrosive, but not as much as bleach. Baking soda may be a better alternative as it is still alkaline, but with a pH of 8 to 9 is not considered corrosive.
On the other end of the pH scale at a pH of 3 is vinegar, an acidic cleaner that many believe to be the perfect all-purpose cleaner. While it’s great at removing mineral deposits, it can corrode surfaces. Do not use vinegar on wood, stone, waxed surfaces, plants, your dishwasher, knives, washing machine, small appliances or electronic screens.
For more information contact us!


 by Autumn Ryan, Founder and CEO, Aseptic Health
by Autumn Ryan, Founder and CEO, Aseptic Health

 by Dana Ryan, Certified Clinically Clean® Specialist
by Dana Ryan, Certified Clinically Clean® Specialist

 3 Strikes And Your Out
3 Strikes And Your Out
 by Pat Cummings, Certified Clinically Clean® Specialist
by Pat Cummings, Certified Clinically Clean® Specialist From Andrea Velasquez, Certified Clinically Clean® Specialist
From Andrea Velasquez, Certified Clinically Clean® Specialist
 by Autumn Ryan, Founder and CEO
by Autumn Ryan, Founder and CEO



