How It Works?
DISINFECTS AT THE MICROSCOPIC LEVEL
The effectiveness of SoRite stems from the potent oxidizing ions that are contained in Sodium Chlorite, the primary active ingredient. Disinfectants rely on oxidizing agents to kill microorganisms. Sodium Chlorite is a much more potent oxidizing agent than the oxidizing agents contained in other disinfectants.
Sodium Chlorite oxidizes and destroys proteins and nucleic acids, DNA and RNA, that are key to the survival of pathogenic microorganisms that include viruses, bacteria and fungi. The effectiveness of SoRite stems from the fact that it can rapidly penetrate the cell walls of microorganisms, disrupt proteins and nucleic acids, thus killing the microorganisms.
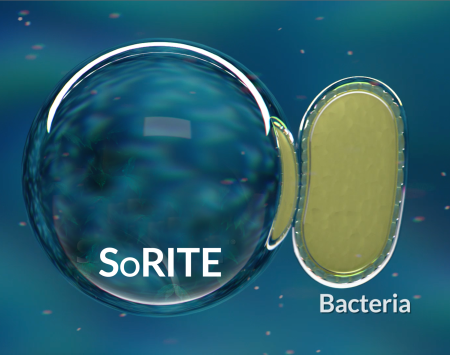


SoRite droplet begins to penetrate bacteria.
SoRite contains the active ingredient Sodium Chlorite.
Bacteria cell wall inside SoRite droplet begins degrading bacteria cell wall. Sodium Chlorite rapidly breaks down the cell walls of bacteria.
Bacteria is destroyed. Cell walls are disrupted, which ultimately kills bacteria.

SoRite is the first to use Sodium Chlorite in such low concentrations— achieving the lowest toxicity category by the EPA—yet the fastest kill time, making it a superior antimicrobial agent. It’s this technology that allows SoRite to be so effective and still safe to use without protective equipment. There truly is no comparison when it comes to disinfectants.


